Introduction: Patients (pts) with relapsed or refractory ( R/ R) large B-cell lymphoma (LBCL) after first-line treatment who are unable to undergo consolidative high-dose chemotherapy (HDCT) and hematopoietic stem cell transplantation (HSCT) have poor outcomes and limited treatment options. Rituximab, gemcitabine and oxaliplatin (R-GemOx) is a common immuno-chemotherapy regimen used in this setting. The objective of the open-label, phase 3, NIVEAU study (NCT03366272) was to evaluate the efficacy and safety of Nivolumab added to R-GemOx in pts with R/ R LBCL after 1 prior line of therapy not eligible for HDCT/HSCT owing to age and/or comorbidities.
Patients and Methods: Eligible pts were adults with R/R LBCL that were refractory to or had relapsed after first-line immuno-chemotherapy and who were not deemed candidates for HDCT/HSCT based on physician's assessment and at least one of the following criteria: age ≥65 years; age ≥18 years and Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) score ≥3; or age ≥18 years and prior ASCT (as 1st line consolidation). Therapy consisted of 8 cycles R-GemOx (arm A) or 8 cycles of nivolumab plus R-GemOx followed by nivolumab as maintenance for up to 1 year in total or until progression or unacceptable toxicity (arm B). The primary endpoint was progression-free survival (PFS) after 1 year. Major secondary endpoints were response rates, event-free- (EFS) (time to progression, relapse, death or unplanned anti-lymphoma treatment), overall survival (OS) and toxicity. The protocol specified a pre-planned interim analysis after the first 180 pts completed up to at least 1 follow-up investigation.
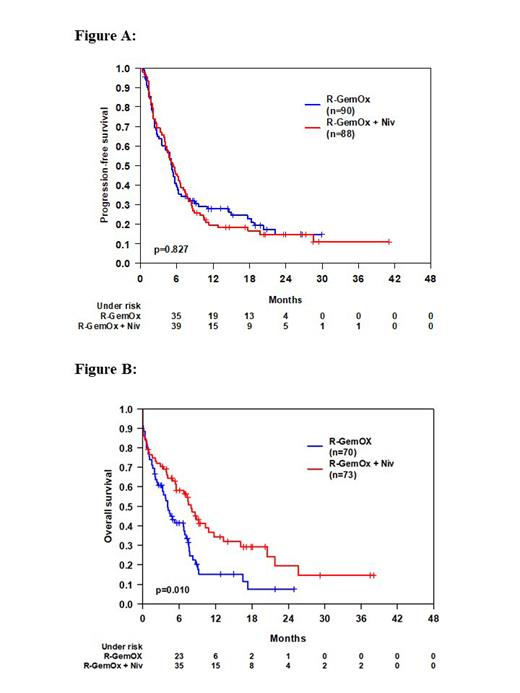
Results: Between Jan 2018 and May 2021, 180 pts were randomized. Two pts did not receive any treatment (one withdrawn informed consent, one death) resulting in a full analysis set of n=90 (arm A) and n=88 (arm B). Median age was 76 years (range 44-87), 52% of pts were >75 years of age, 37% were primary progressive after first-line therapy, 62% had IPI 3-5. Overall response (OR) and complete response (CR) rates were similar with 34% and 20% (arm A) vs. 34% and 22% (arm B), respectively. Rates of progressive disease (PD) were 53% (arm A) vs. 50% (arm B). After a median follow-up of 21 months, 1-year PFS was 28% (95%-CI: 18%-37%) in arm A vs. 20% (95%-CI: 11%-28%), (p=0.827) in arm B (Figure A). 1-year EFS was 22% (95%-CI: 13%-31%) in arm A vs. 20% (95%-CI: 11%-28%), (p=0.654) in arm B. One-year OS was 48% (95%-CI: 38%-59%) in arm A vs. 58% (95%-CI: 47%-68%), (p=0.126) in arm B. At 2 years, PFS, EFS and OS were 15% (95%-CI: 6%-24%), 13% (95%.CI: 5%-21%) and 34% (95%-CI: 22%-46%) in arm A vs. 15% (95%-CI: 7%-23%), 15% (95%-CI: 7%-23%) an 43% (95%-CI: 32%-55%) in arm B. Because of an identical PFS but a trend toward better OS for arm B, we performed an event-driven OS analysis of pts after second progression or relapse (n=70 in arm A, n= 73 in arm B). OS survival was superior in arm B vs. arm A after second progression or relapse (Log Rank p= 0.010) (Figure B). There was a small increase in number of pts with grade 3-5 AEs in arm B (arm A/arm B: thrombocytopenia 37%/39%; anemia 19%/26%; leukopenia 19%/24%; infection 15%/22%). No increase in number of pts with immune-related grade 3-5 AEs was observed except asymptomatic increase of lipase (arm A/arm B: 23%/27%), (p=0.625) and amylase (arm A/arm B: 5%/11%), (p=0.192). Most common grade 3-5 AEs during nivolumab consolidation in arm B were leukopenia 13%, peripheral neuropathy 10%, increase of lipase 8%, anemia 5% and infection 5% of pts). In total, 7 treatment-related deaths occurred (arm A/arm B: 3 (3%)/4 (5%).
After this interim analysis revealed futility to demonstrate superiority for the primary endpoint, recruitment was stopped in March 2023. At that point, 270 of 310 initially intended pts had been recruited.
Conclusions: In pts with R/R LBCL, the addition of nivolumab to R-GemOx followed by nivolumab maintenance is feasible without relevant additive toxicity. The addition of Nivolumab did not improve PFS of the R-GemOx regimen alone. These results demonstrate the poor prognosis of R/R transplant-ineligible pts treated with immuno-chemotherapy. However, a trend towards a better OS was observed, which allows for the hypothesis that nivolumab might enhance the efficacy of subsequent therapies. The final analysis of the study is scheduled after completion of follow-up of the last patient included as defined by the study protocol.
OffLabel Disclosure:
Held:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Kerkhoff:BeiGene, BMS, pharma: Honoraria; Roche,Sobi: Honoraria; AbbVie, Amgen, Zeneca: Honoraria; Takeda: Honoraria. Casasnovas:Abbvie: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; AMGEN: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; BEIGENE: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GILEAD/KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ROCHE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tilly:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Da Silva:Astra Zeneca: Research Funding; Roche, Janssen Cilag, Gilead Sciences; AbbVie, Takeda: Consultancy. Tonino:Roche: Honoraria; Takeda: Honoraria; InCyte: Honoraria; Beigene: Research Funding. Dreyling:Abbvie, Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Other: Scientific advisory boards; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding; Astra Zeneca, Beigene, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria. Safar:janssen: Honoraria. Mayer:Amgen, Abbvie: Other: travel grants; Amgen, Novartis Roche: Honoraria. Morschhauser:F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy. Scholz:GILEAD: Honoraria, Other: consulting fees; Incyte: Other: consulting fees; Janssen Cilag: Honoraria, Other: consulting fees; Novartis: Other: consulting fees; Roche: Honoraria, Other: consulting fees, travel support; Takeda: Honoraria, Other: consulting fees, travel support; GenMab: Other: consulting fees. Dabrowska-Iwanicka:Abbvie: Other: Travel grants; Gilead: Other: Travel grants. Gaulard:Takeda: Consultancy; Roche: Other: travel and congress support; Sanofi: Research Funding. Molina:Janssen: Other: Travel and congress fees. Greil:Roche: Honoraria, Research Funding. Jaeger:Innovative Medicines Initiative 2 Joint Undertaking: Research Funding; BMS, Novartis, Gilead, Miltenyi, Janssen and Roche: Honoraria. Avigdor:BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel/Accommodations/Expenses; Takeda: Membership on an entity's Board of Directors or advisory committees; MSD: Research Funding. Poeschel:AstraZeneca: Honoraria; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, congress support; EUSA Pharma: Consultancy; Janssen-Cilag: Consultancy; Roche: Other: travel expenses, congress support; Genmab: Consultancy; Abbvie: Other: travel expenses, congress support; Amgen: Other: travel expenses, congress support; PentixaPharm GmbH: Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum GmbH: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, congress support; Lilly: Membership on an entity's Board of Directors or advisory committees. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria.
Nivolumab